Qualifications and Validations (IQ, OQ) of Pharmaceutical Usage Equipment According to GMP
Sartorom's technical department offers validation services for equipment used in the pharmaceutical industry in accordance with GLP/GMP requirements and regulations.
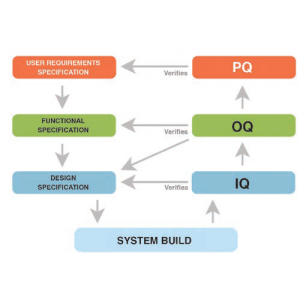
Qualification consists of performing specific tests and drawing up the related validation protocols: IQ; OQ for:
– Dissolution systems; disintegration testers; hardness, friability, abrasion, powder flow testers; bulk density testers; R&D equipment;
– autoclaves, incubators, climatic/stability chambers, ovens and freezers;
– materials testing machines;
– weighing apparatus; balances, thermogravimeters;
– titrators, pH meters, oxygen meters, etc.
– laser and video granulometers;
– thermal analysis equipment.

Reviews
There are no reviews yet.